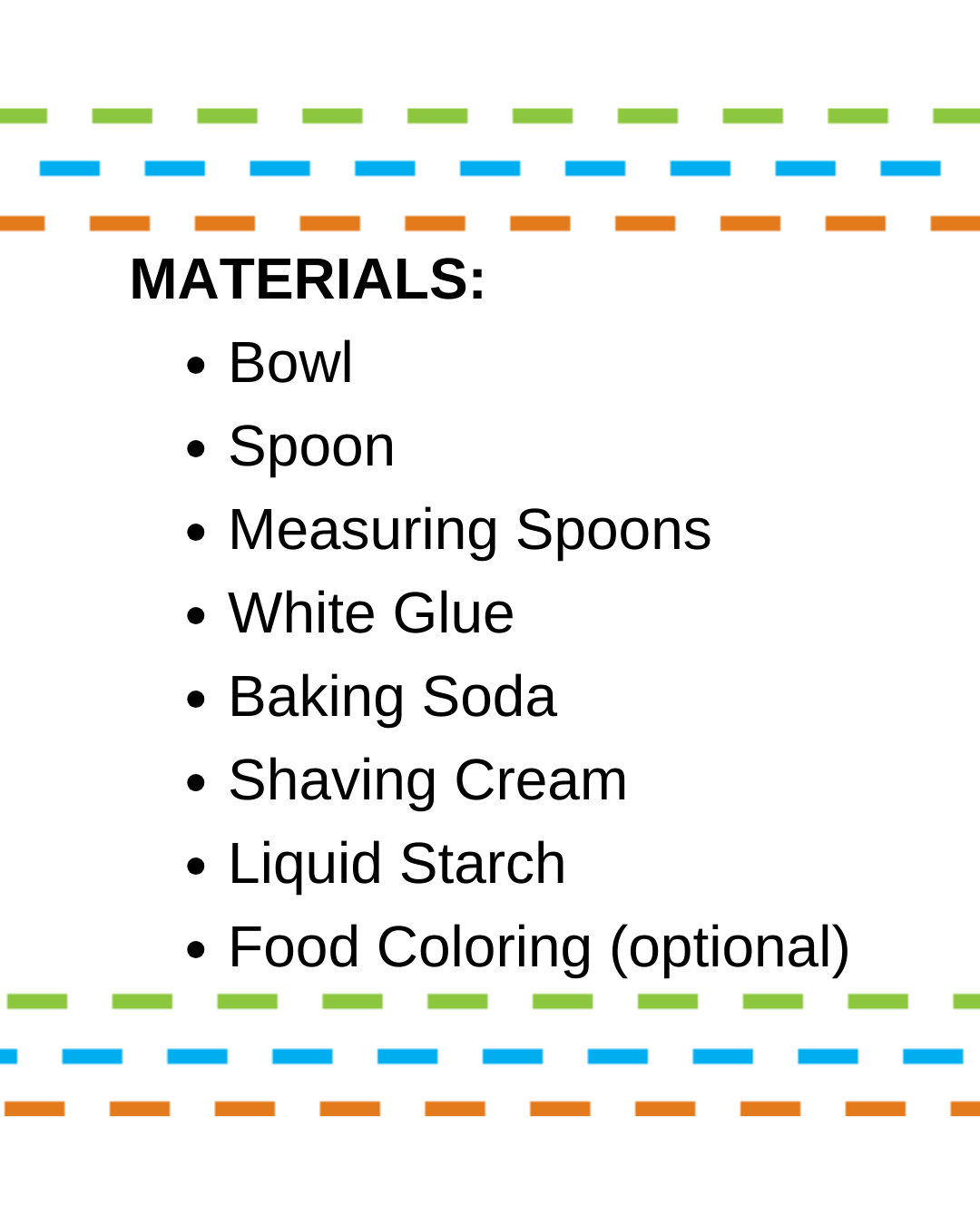
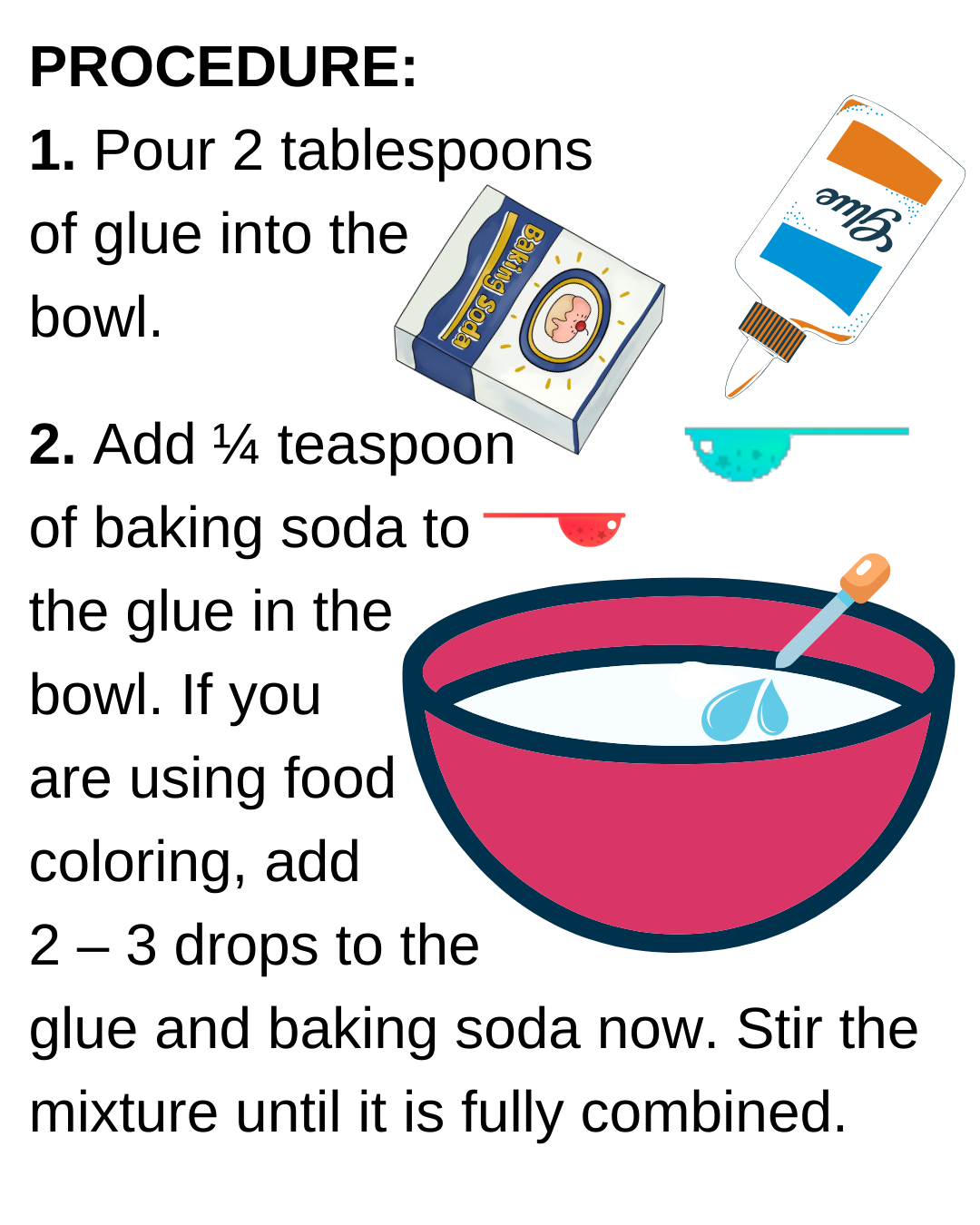
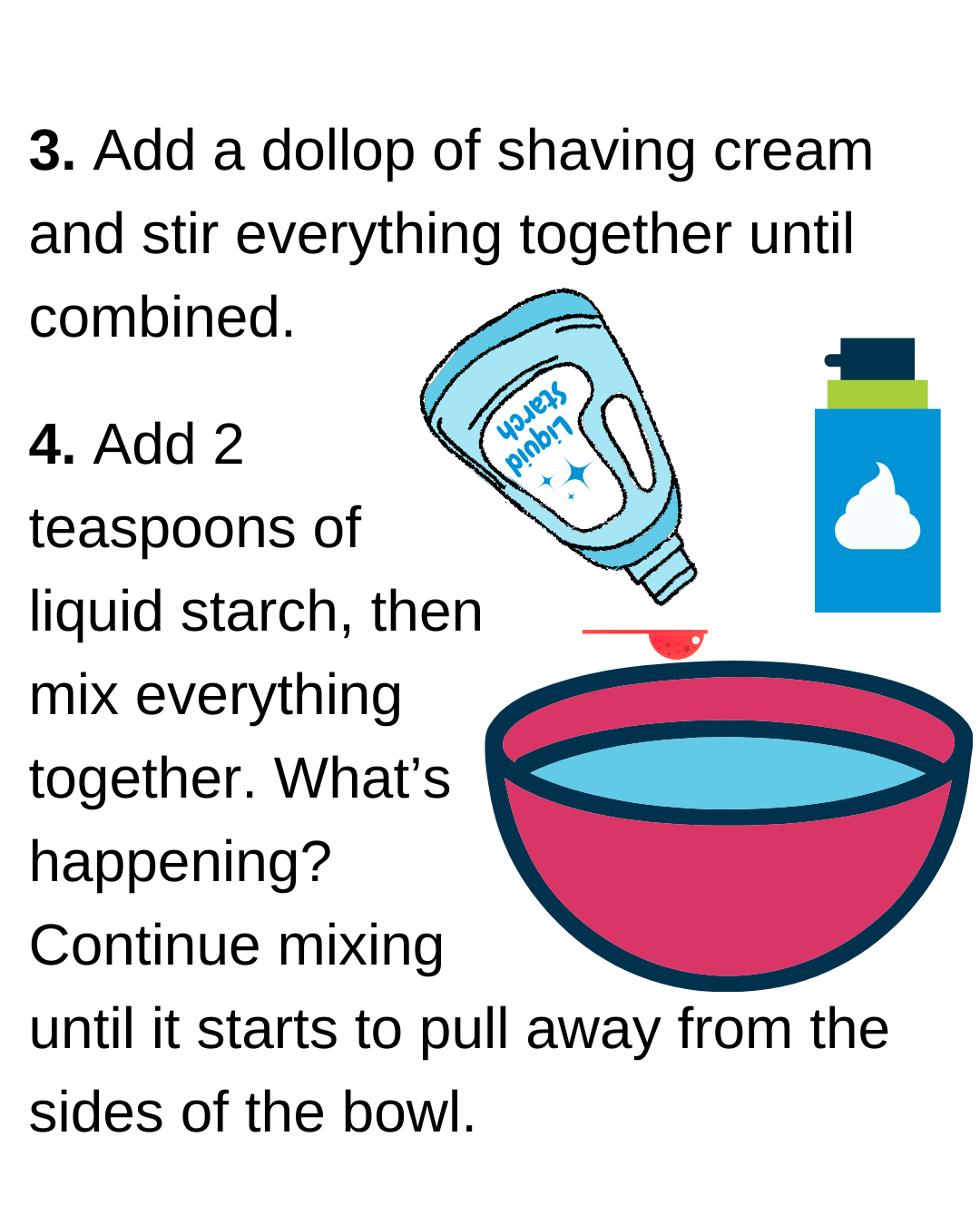
Open every weekend for timed-entry sessions!


Northern Virginia Science Center Foundation is a 501(c)(3) non-profit organization that operates the Children's Science Center Lab at Fair Oaks Mall and STEM programs traveling to schools and other community venues across the region. The Foundation is also developing the Northern Virginia Science Center in Dulles, VA, a world-class, interactive regional science center for families, students and learners of all ages made possible through a pivotal public-private partnership. Learn more about our mission today at childsci.org and our vision for the future at novasci.org.